
Doptelet provides a rapid and durable platelet count lift that lasts1*†
Pivotal trial:
Primary Endpoint: Patients on Doptelet obtained a platelet response for a median of 12.4 cumulative weeks without the need for rescue therapy.1‡§II
Secondary Endpoint:
Secondary Endpoint:
Day 8 Platelet Count ≥50x109/L1¶
Core study (pivotal trial)
Efficacy was evaluated in a 6-month, multicenter, randomized, double-blind, placebo-controlled Phase 3 study. Patients had received one or more prior chronic ITP therapies and had average screening and baseline platelet counts of <30×109/L. Forty-nine patients were randomized (2:1) to receive either Doptelet (n=32) or placebo (n=17).1
- The primary efficacy endpoint was the cumulative number of weeks of platelet response, defined as a platelet count ≥50×109/L in the absence of rescue therapy, over 6 months of once-daily treatment in adults with chronic ITP. Doptelet-treated patients had a median duration of 12.4 cumulative weeks vs 0 weeks for placebo1
- A secondary efficacy endpoint was the proportion of patients with a platelet response (platelet counts ≥50×109/L) at Day 8. 66% (n=21/32) of Doptelet-treated patients had platelet counts of ≥50,000/μL at Day 8 compared to placebo (n=0/17)1
Open-label extension
Patients could enter the open-label extension phase if they completed the 6-month core study, or if they experienced a lack of efficacy during that period. In the extension phase, all patients received titrated Doptelet once daily. Thirty-nine patients (24 Doptelet and 15 placebo) entered the 90-week maintenance period of the extension phase, in which Doptelet dose titration and downward titration of concomitant ITP medications were allowed. At the end of the extension phase, a 4-week, dose-tapering period was followed by a 30-day follow-up after the last dose of Doptelet.3,4
- The primary endpoint of the extension study was to assess the long-term safety and efficacy of treatment with Doptelet by measuring platelet response rate, bleeding, and the use of rescue therapy3
- The secondary efficacy endpoint included the percentage of patients who achieved platelet counts ≥50,000/μL or ≥100,000/μL at any time during the core study and its extension phase. These endpoints were reported in an integrated analysis of the Phase 3 core study and extension phase data. In an integrated analysis of the core study and its extension phase, 93.8% of patients initially randomized to Doptelet and who continued to be treated with Doptelet during the extension phase achieved a platelet count of ≥50,000/μL at any time, compared to 64.7% of placebo patients who rolled over to Doptelet2,4
Post hoc analysis
This post hoc analysis assessed the endpoints from the Phase 3 study and provided previously unreported data on the percentage of patients who were able to achieve a platelet response or complete response at any time during the core study and extension phase.2
Limitations and disclosures*
- The open-label extension was not placebo-controlled; therefore, causality cannot be attributed, and hypothesis testing cannot determine whether within-arm changes were due to drug effect. This data is not included in the Doptelet Prescribing Information
- There is no comparator in the post hoc analysis, and further studies are needed to validate these results. The post hoc analysis is not included in the Doptelet Prescribing Information, and the FDA did not consider this analysis in approving Doptelet
Platelet counts over time
A durable platelet response was observed.1*†
Exploratory endpoint: Mean platelet count from baseline to 6 months for Doptelet-treated vs placebo-treated patients5
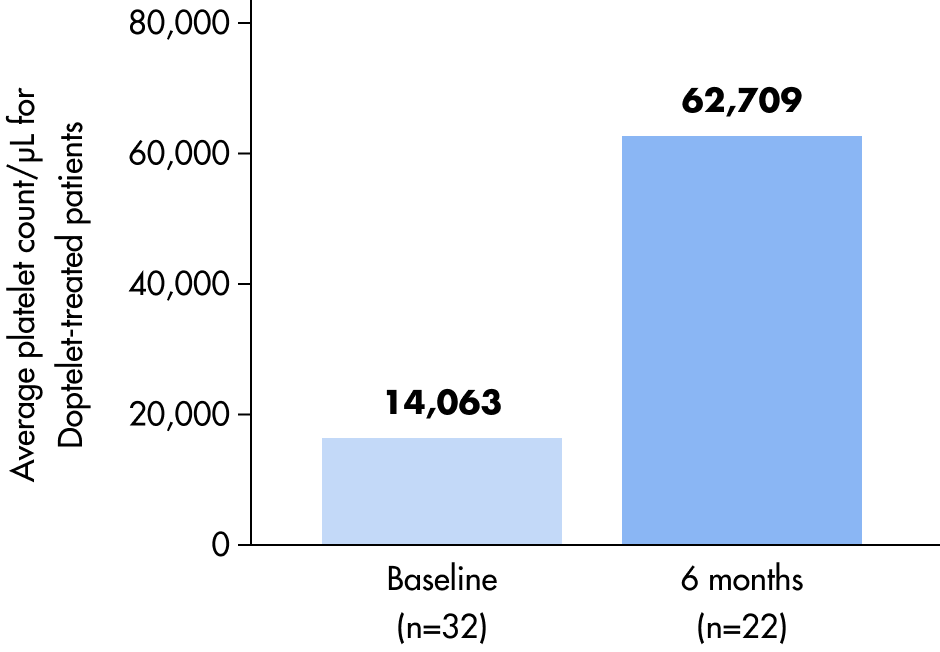
Placebo-treated patients
Seventeen patients that received placebo entered with a mean platelet count of 12,712/μL.
One placebo patient finished the core study with a mean platelet count of 31,000/μL.5
Important disclaimers: This exploratory endpoint is not included in the Doptelet Prescribing Information. The results of this exploratory endpoint may differ from those observed in clinical practice.
FDA=Food and Drug Administration; ITP=immune thrombocytopenia.
Post hoc analysis:
Platelet response across the core and extension study2#**
Across the core study and open-label extension, Doptelet continued to support platelet response in most patients.
94% of patients treated with Doptelet achieved a platelet response at any time.
See how Dr Nagalla interprets these results from the Doptelet clinical trial
The healthcare professional in this video has been compensated by Sobi for these discussions; however, the views expressed during this video may reflect personal opinions and experiences of the healthcare professional. Any patient cases discussed represent individual cases and are not representative of every patient's treatment journey. Individual results may vary.
Observed platelet response based on prior TPO-RA use6#
In a post hoc analysis of the Phase 3 trial, platelet response with Doptelet was evaluated in relation to prior TPO-RA use, and the outcomes were:
- 38% of patients treated with Doptelet had been on a previous TPO-RA therapy
- Comparable efficacy was observed, with median cumulative weeks of platelet counts ≥50×109/L being 12.7 in patients with prior TPO-RA use (n=12) and 12.4 in those without prior TPO-RA use (n=20)
- Up to 6 months of Doptelet exposure in the trial
Median cumulative weeks with platelet response in Doptelet-treated patients by TPO-RA treatment history6
This analysis evaluated platelet response duration in patients previously treated with a TPO-RA versus those who were TPO-RA naive, offering insight into Doptelet performance across different treatment backgrounds.
Important disclamers: This post hoc analysis may not meet the FDA definition of an adequate and well-controlled study. Results from this analysis may differ from those observed in clinical practice. This analysis is not included in the Doptelet Prescribing Information and the FDA did not consider it in approving Doptelet.
FDA=Food and Drug Administration; TPO-RA=thrombopoietin receptor agonist.
Observational study:
Data in patients initiating treatment with Doptelet
A multicenter, observational study was conducted in adult patients with primary or secondary ITP who changed from eltrombopag or romiplostim to Doptelet for any reason. Patients received Doptelet for at least 2 months. The primary endpoint was platelet response at least once without the need for rescue therapy.7
In the study population7:
- 93% (41/44) achieved a platelet response (≥50,000/μL)
- 86% (38/44) achieved a complete response (≥100,000/μL)
- 63% (12/19) of those previously on chronic steroids were able to stop steroid use after initiating Doptelet
Study limitations
- This was a retrospective observational study and was subject to limitations of such a study, such as selection bias and potential confounding, as well as the lack of a defined treatment protocol resulting in heterogeneity of dose adjustment and follow-up frequency. There were relatively low patient numbers for each subgroup analysis. Non-platelet outcomes were not collected, including bleeding events and health-related quality of life
- It is not known if patients maintained on romiplostim or eltrombopag would have spontaneously improved their platelet counts had those agents been continued rather than switched to avatrombopag. Hence, these results are not intended to support conclusions of superior efficacy or safety
- This study is not included in the Doptelet Prescribing Information and the FDA did not consider it in approving Doptelet
FDA=Food and Drug Administration; ITP=immune thrombocytopenia.
The Doptelet safety data
Review adverse events and key safety takeaways from clinical use.
- DOPTELET (avatrombopag) [prescribing information]. Morrisville, NC: AkaRx, Inc; 2025.
- Nagalla S, Vredenburg M, Tian W, Allen LF. Platelet response to avatrombopag in patients with chronic immune thrombocytopenia: additional analyses from a phase 3 study and its extension. Blood. 2019;134(suppl 1):1071.
- Jurczak W, Chojnowski K, Mayer J, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol. 2018;183(3):479-490.
- Al-Samkari H, Aggarwal K, Vredenburg M, Tian W, Allen LF. Long-term response rates in patients with chronic immune thrombocytopenia treated with avatrombopag: additional analyses from a phase 3 study and its extension phase. Blood. 2019;134(suppl 1):2356.
- Data on file. 302 clinical study report. 2016: Sobi, Inc.
- McCrae KR, Allen LF, Aggarwal K, Vredenburg M, Tian W, Kuter D. Poster presented at: ISTH Conference 2019. Abstract PB0416.
- Al-Samkari H, Jiang D, Gernsheimer T, Liebman H, Lee S, Wojdyla M, Vredenburg M, Cuker A. Adults with immune thrombocytopenia who switched to avatrombopag following prior treatment with eltrombopag or romiplostim: A multicentre US study. Br J Haematol. 2022;197(3):359-366.