
When first-line treatments fall short, TPO-RAs step in
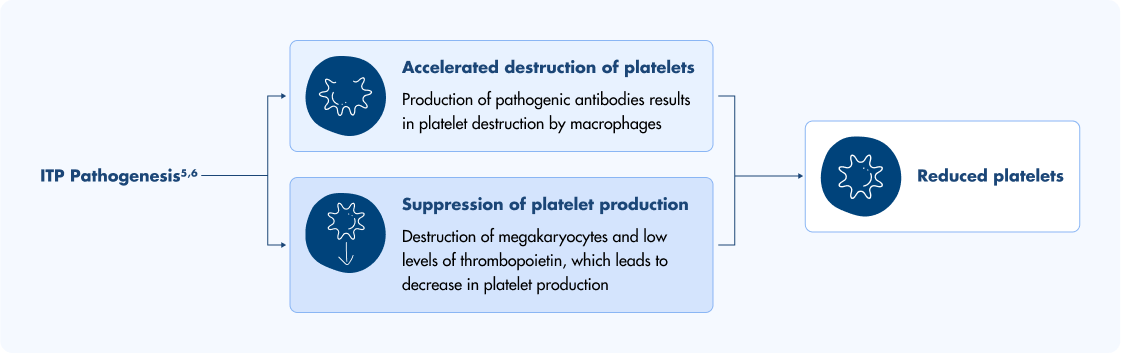
For adults with chronic immune thrombocytopenia (ITP), a condition characterized by increased platelet destruction and impaired platelet production, second-line treatments can be considered when first-line treatments are no longer effective. TPO-RAs like Doptelet activate the TPO receptor to increase platelet production and help manage chronic ITP. Doptelet is not used to make platelet counts normal.1,4II
The safety of Doptelet has been studied in a combined 7+ years across at least 17,000 adult and pediatric patients1,7-14¶
Why choose a TPO-RA to treat ITP?
The ASH guidelines recommend a short course of ≤6 weeks of steroids when prescribing for newly diagnosed adults with ITP.4
Could Doptelet be right for your patients?
Explore real case profiles and see how HCPs approach these same cases in the Platelet Couch video series.
What makes Doptelet different
ASH guidelines recommend selecting treatment options that align with patient preferences, which can play a key role in adherence.4
No food-type restrictions
Patients can take Doptelet with any food (food required); no administration concerns with minerals like calcium or magnesium.1
No liver monitoring or cataract precautions
Doptelet does not require additional liver function monitoring or eye exams before or during treatment. No significant hepatotoxicity was seen in clinical trials.1,8,16
No treatment injections
Doptelet is an oral medication that can be taken anytime, anywhere—without adding trips to the doctor.1‡
Remember to monitor drug interactions and platelet response.1‡
JAK-STAT=Janus kinase-signal transducer and activator of transcription; TPO=thrombopoietin.
How Doptelet works
Doptelet stimulates platelet production without blocking native TPO.1
- Doptelet is an oral TPO-RA that binds to the c-Mpl (TPO) receptor on hematopoietic stem cells1,5,16
- Its binding site is distinct from endogenous TPO and other TPO-RAs, activating different signal transduction pathways1,5,16
- This results in JAK-STAT pathway activation, megakaryocyte maturation, and increased platelet production1,5,16
- Because it doesn’t compete with native TPO, Doptelet allows for an additive effect on platelet counts1,16
- This differentiated signaling profile may offer an alternative approach for patients transitioning from other TPO-RAs16
Where would you like to go next?

I'm considering Doptelet for my adult patients with chronic ITP

I’m reviewing how to dose Doptelet

I’m starting a patient on Doptelet and looking for patient and financial support
I’m looking to prescribe Doptelet or Doptelet Sprinkle for a pediatric patient

- DOPTELET (avatrombopag) [prescribing information]. Morrisville, NC: AkaRx, Inc; 2025.
- Nplate (romiplostim) [prescribing information]. Thousand Oaks, CA: Amgen; 2025.
- Promacta (eltrombopag) [prescribing information]. East Hanover, NJ: Novartis Pharmaceuticals Corp; 2023.
- Neunert C, Terrell DR, Arnold DM, et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3(23):3829-3866.
- Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6(2):16.
- Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129(21):2829-2835.
- Data on file. June 3, 2025: Sobi, Inc.
- Jurczak W, Chojnowski K, Mayer J, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. Br J Haematol. 2018;183(3):479-490.
- FDA approves avatrombopag for thrombocytopenia in adults with chronic liver disease. U.S. Food & Drug Administration. May 21, 2018. Accessed July 24, 2025. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-avatrombopag-thrombocytopenia-adults-chronic-liver-disease
- Sobi announces FDA acceptance of new drug application for avatrombopag (DOPTELET®) for the treatment of pediatric immune thrombocytopenia. News release. Biospace. December 12, 2024. Accessed July 24, 2025. https://www.biospace.com/press-releases/sobi-announces-fda-acceptance-of-new-drug-application-for-avatrombopag-doptelet-for-the-treatment-of-pediatric-immune-thrombocytopenia
- Fala L. Doptelet (avatrombopag) receives FDA approval for the treatment of patients with chronic immune thrombocytopenia. American Health & Drug Benefits®. Updated August 30, 2021. Accessed July 24, 2025. https://ahdbonline.com/issues/2019/2019-payers-guide-mid-year-addendum/doptelet-avatrombopag-receives-fda-approval-for-the-treatment-of-patients-with-chronic-immune-thrombocytopenia
- ClinicalTrials.gov. Treatment of thrombocytopenia in patients with chronic liver disease undergoing an elective procedure (NCT01972529). Accessed July 25, 2025. https://clinicaltrials.gov/study/NCT01972529
- ClinicalTrials.gov. Efficacy and safety of oral E5501 plus standard of care for the treatment of thrombocytopenia in adults with chronic immune thrombocytopenia (NCT01438840). Accessed July 25, 2025. https://clinicaltrials.gov/study/NCT01438840
- ClinicalTrials.gov. Avatrombopag for the treatment of thrombocytopenia in pediatric subjects with immune thrombocytopenia for ≥6 months (NCT04516967). Accessed July 25, 2025. https://clinicaltrials.gov/study/NCT04516967
- Provan D, Arnold DM, Bussel JB, et al. Updated international consensus report on the investigation and management of primary immune thrombocytopenia. Blood Adv. 2019;3(22):3780-3817.
- Kuter DJ. The structure, function, and clinical use of the thrombopoietin receptor agonist avatrombopag. Blood Rev. 2022;53:100909.