
Doptelet and Doptelet Sprinkle provided a rapid and durable platelet count lift1*†
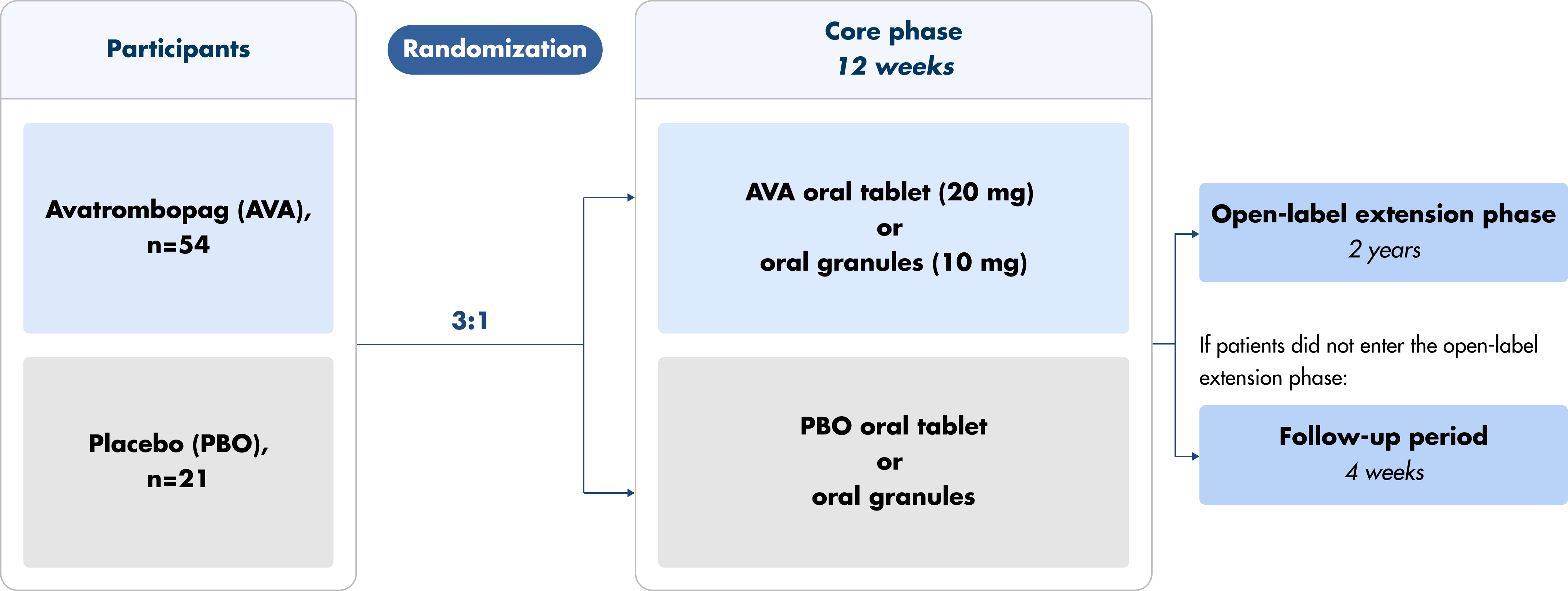
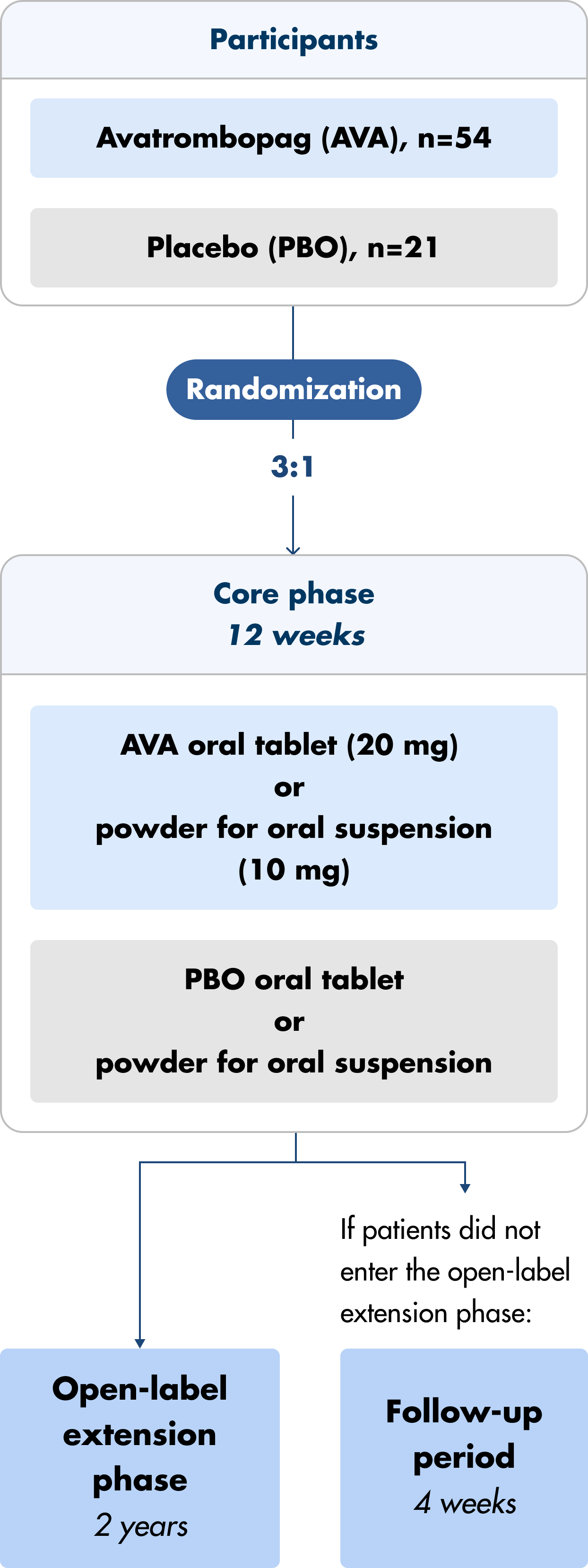
Doptelet and Doptelet Sprinkle were studied in a multicenter, randomized, double-blind, placebo-controlled, parallel group trial.1,2
- Core phase (12 weeks) and extension phase (2 years)
- Patients were administered either the oral tablet (ages ≥6 to <18 years) or oral granules (ages ≥1 to <6 years) daily
- Patients were randomized 3:1, Doptelet arm with 54 patients and placebo arm with 21 patients
Patients included in the study were pretreated and had varying experiences with TPO-RAs. Baseline characteristics were balanced between the treatment arms. For both groups1-3‡:
- Patients who had received ≥3 ITP treatments
- 68.5% of patients receiving Doptelet or Doptelet Sprinkle
- 66.7% of patients receiving placebo
- Patients who had received at least one prior TPO-RA
- 74.1% of patients receiving Doptelet or Doptelet Sprinkle
- 71.4% of patients receiving placebo
- Patients who had not previously responded to a TPO-RA
- 57.5% of patients receiving Doptelet or Doptelet Sprinkle (investigators’ assessment)§
- 80% of patients receiving placebo
Note: Patients had the option to be removed from the core phase and enrolled in the open-label extension due to lack of treatment effect. Previous therapy with any other TPO-RA or recombinant human TPO must have been completed at least 28 days before Day 1.3
| AVA (n=54) | PBO (n=21) | |
|---|---|---|
| Female, n (%) | 24 (44.4) | 12 (57.1) |
| Age, years (mean ± SD) | 8.9 ± 4.4 | 9.9 ± 4.1 |
| Platelet count ≤15×10⁹/L, n (%) | 45 (83.3) | 17 (81.0) |
| Platelet count, median (IQR) | 10.4 (8.0–15.0) | 11.5 (6.0–15.5) |
| Bruising or bleeding (Grades 1 & 2), n (%) | 39 (72.2) | 16 (76.2) |
WHO bleeding scale for the 7 days prior to baseline, n (%)
| 36 (66.7) 3 (5.6) | 14 (66.7) 2 (9.5) |
| Time from primary ITP diagnosis to first dose, years (mean ± SD) | 3.87 ± 3.15 | 4.32 ± 3.48 |
The Doptelet and Doptelet Sprinkle arm had 2x treatment duration vs placebo2
Median treatment duration
Placebo patients had the option to switch to Doptelet or Doptelet Sprinkle and enroll in the open-label extension, leading to a lower treatment duration for the placebo arm.
of patients treated with Doptelet or Doptelet Sprinkle achieved a durable platelet response (a platelet response for ≥6 of the last 8 weeks of treatment) in the absence of rescue therapy vs 0% of placebo patients (primary endpoint)1,3,4‡II
of patients treated with Doptelet or Doptelet Sprinkle demonstrated a platelet response in the absence of rescue therapy vs 0% of placebo patients (alternative primary endpoint analyzed as a secondary endpoint)1,3,4‡II¶
Median platelet counts in the absence of rescue therapy (core phase)1,3,4
Scroll left and right to view the full chart.
of Doptelet and Doptelet Sprinkle patients experienced a platelet response of ≥30,000 platelets per microliter for at least 6 of the last 8 weeks.4#**
rescue therapy (secondary endpoint)#††
of placebo patients†
Primary endpoints3
- Durable platelet response: Proportion of patients achieving at least 6 out of 8 weekly platelet counts ≥50×109/L during the last 8 weeks of the 12-week core phase treatment period in the absence of rescue therapy
- Platelet response (alternate primary endpoint analyzed as a secondary endpoint): Proportion of patients for whom at least 2 consecutive platelet assessments were ≥50×109/L over the 12-week core phase treatment period in the absence of rescue therapy
Select secondary endpoints1,3
- Platelet response at Day 8: Defined by the proportion of patients with a platelet count ≥50×109/L at Day 8, in the absence of rescue therapy
- Rescue therapy use: Proportion of patients who required rescue therapy during 12 weeks of treatment
#Platelet response is defined by the proportion of patients with a platelet count of ≥50x109/L.3
AVA=avatrombopag; IQR=interquartile range; ITP=immune thrombocytopenia; PBO=placebo; SD=standard deviation; TPO=thrombopoietin; TPO-RA=thrombopoietin receptor agonist; WHO=World Health Organization.

See the safety data for Doptelet and Doptelet Sprinkle
Review adverse events and key safety takeaways.
Connect with an expert
Get guidance on starting treatment, managing access, and supporting your patients taking Doptelet or Doptelet Sprinkle.
- DOPTELET (avatrombopag) [prescribing information]. Morrisville, NC: AkaRx, Inc; 2025.
- Data on file. Clinical study report for AVA-PED-301. 2025: Sobi, Inc.
- Grace RF, Leblebisatan G, Aydinok Y, et al; on behalf of the AVA-PED-301 Study Group. Avatrombopag for the treatment of children and adolescents with immune thrombocytopenia (AVA-PED-301): a multicentre, randomised, double-blind, placebo-controlled, phase 3b study. Lancet Haematol. 2025;12(7):e494-e504.
- Grace RF, Leblebisatan G, Aydinok Y, et al. Analysis of durability of response to avatrombopag (AVA) from a phase 3b multicenter, randomized, double-blind, placebo (PBO)-controlled, parallel-group trial to evaluate the efficacy and safety of AVA for the treatment of pediatric patients with immune thrombocytopenia. Poster presented at: ASH Annual Meeting; December 7-10, 2024; San Diego, CA. Session 311.