
ADAPT‐1 and ADAPT‐2 were 2 identically designed, multicenter,
randomized, double‐blind, placebo‐controlled studies (N=435)1,2
Primary endpoint:
Proportion of patients who did not require a platelet transfusion or any rescue procedure for bleeding after randomization and up to 7 days following an elective procedure.1
Secondary endpoint:
Proportion of patients who achieved platelet counts of ≥50x109/L on the day of procedure, and the change in platelet count from baseline to procedure day.1
- Biliary interventions
- Bronchoscopy +/- biopsy
- Chemoembolization for HCC
- Colonoscopy +/- polypectomy/biopsy
- Dental procedures
- Ethanol ablation
- Laparoscopic interventions
- Liver biopsy
- Nephrostomy tube placement
- Paracentesis
- Radiofrequency ablation
- Renal biopsy
- Thoracentesis
- Transjugular intrahepatic portosystemic shunt
- Upper GI endoscopy +/- biopsy
- Upper GI endoscopy +/- sclerotherapy
- Upper GI endoscopy +/- variceal banding
- Vascular catheterization
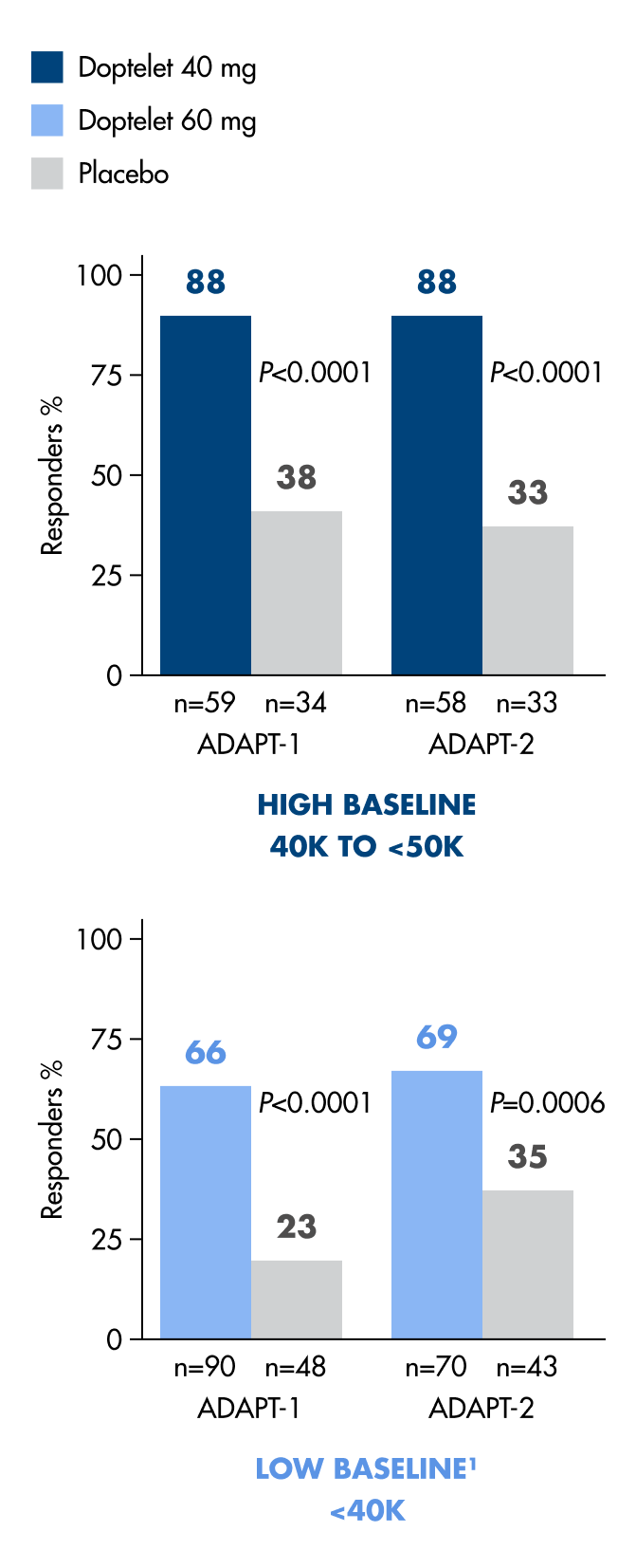
Doptelet helps protect patients during and after a scheduled procedure1
Doptelet significantly reduced the need for platelet transfusions or rescue procedures for bleeding for up to 7 days post procedure.1*
88%
of high-baseline patients taking Doptelet achieved the primary endpoint (N=117).1
66%
of low-baseline patients taking Doptelet achieved the primary endpoint (N=90).1
Mean platelet counts nearly doubled with Doptelet1
With Doptelet, platelet counts significantly increased by procedure day.1
Up to 45,000
in high-baseline patients
Up to 32,000
in low-baseline patients1
Scroll left and right to view the full chart.

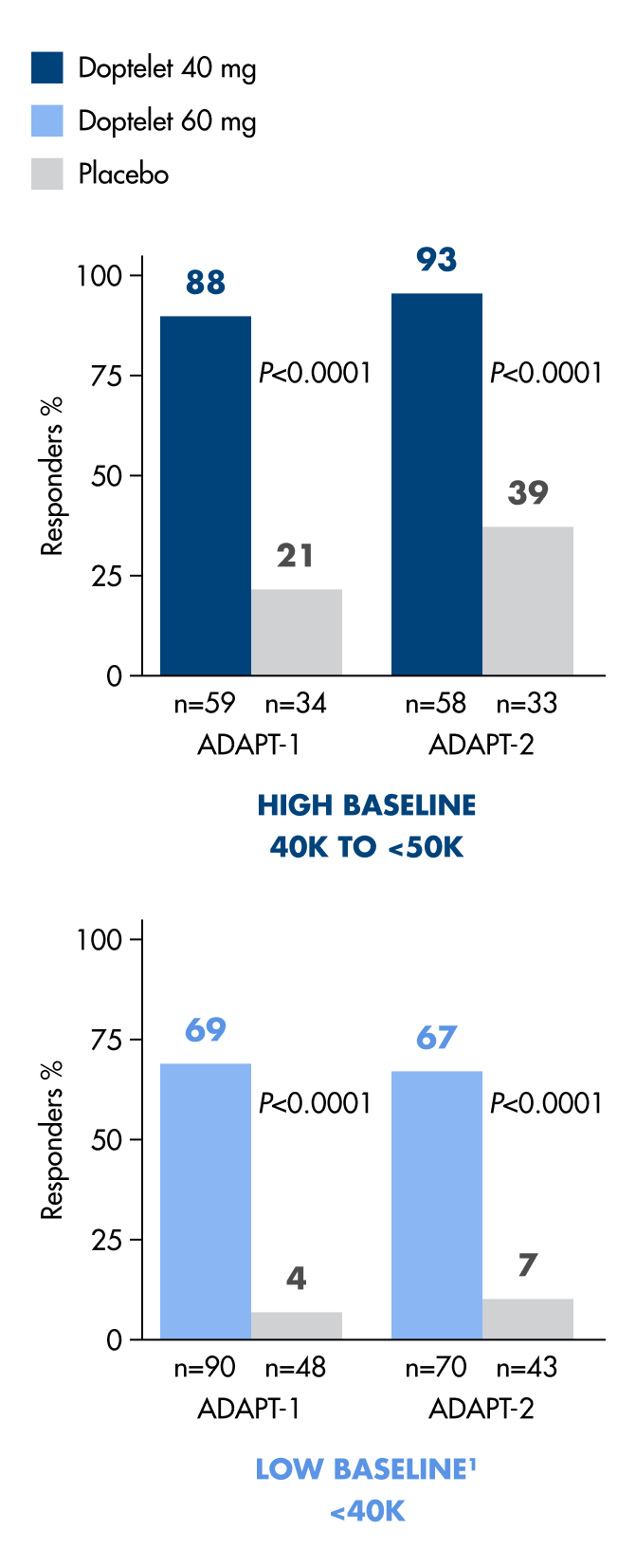
With Doptelet, patients can be ready for procedure day with ≥50,000 platelets/μL1
Significantly more patients achieved a platelet count of ≥50,000/μL with Doptelet.1
93%
of high-baseline patients reached the ≥50,000 platelets/μL target (N=58).1
69%
of low-baseline patients reached the ≥50,000 platelets/μL target (N=90).1
*From randomization of Doptelet 40 mg or Doptelet 60 mg once daily for 5 days up to 7 days after a procedure compared to placebo (P<0.0001; N=435).1
†270/277 patients treated with Doptelet in ADAPT-1 and ADAPT-2 had increased platelet counts by procedure day.1,3
PC=platelet count; qd=every day.
Looking into Doptelet safety data?
Here’s what was observed in clinical trials.
- DOPTELET (avatrombopag) [prescribing information]. Morrisville, NC: AkaRx, Inc;2025.
- Terrault N, Chen Y, Izumi N, et al. Avatrombopag before procedures reduces needs for platelet transfusion in patients with chronic liver disease and thrombocytopenia. Gastroenterology. 2018;155(3):718.
- Data on file. The SAS System. 2023: Sobi, Inc.